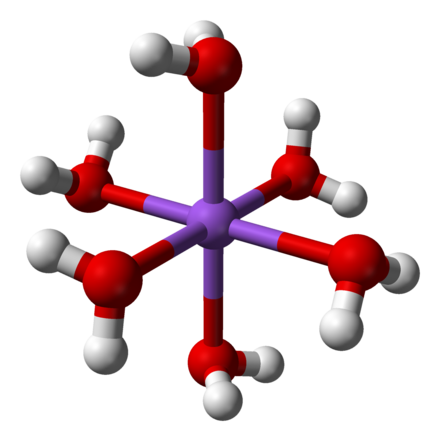
A metal ion in solution does not exist in isolation, but in combination with ligands (such as solvent molecules or simple ions) or chelating groups, giving rise to complex ions or coordination compounds. These complexes contain a central atom or ion, often a transition metal, and a cluster of ions or neutral molecules surrounding it. Ligands are ions or neutral molecules that bond to a central metal atom or ion. Ligands act as Lewis bases (electron pair donors), and the central atom acts as a Lewis acid (electron pair acceptor). Ligands have at least one donor atom with an electron pair used to form covalent bonds with the central atom. The term ligand come from the latin word ligare (which meaning to bind) was first used by Alfred Stock in 1916 in relation to silicon chemistry. Ligands can be anions, cations, or neutral molecules. Ligands can be further characterized as monodentate, bidentate, tridentate etc. where the concept of teeth (dent) is introduced, hence the idea of bite angle etc. A monodentate ligand has only one donor atom used to bond to the central metal atom or ion.

The term "monodentate" can be translated as "one tooth," referring to the ligand binding to the center through only one atom. Some examples of monodentate ligands are: chloride ions (referred to as chloro when it is a ligand), water (referred to as aqua when it is a ligand), hydroxide ions (referred to as hydroxo when it is a ligand), and ammonia (referred to as ammine when it is a ligand).
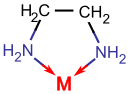
Bidentate ligands have two donor atoms which allow them to bind to a central metal atom or ion at two points. Common examples of bidentate ligands are ethylenediamine (en), and the oxalate ion (ox). Shown below is a diagram of ethylenediamine: the nitrogen (blue) atoms on the edges each have two free electrons that can be used to bond to a central metal atom or ion.
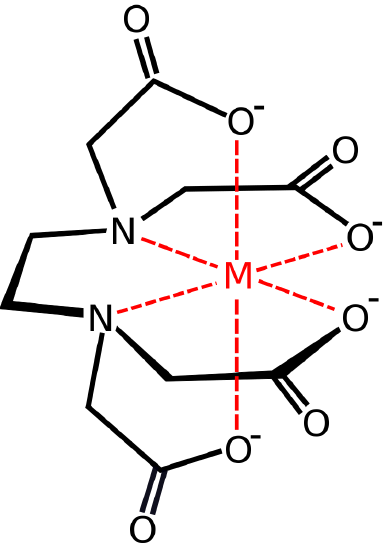
Polydentate ligands range in the number of atoms used to bond to a central metal atom or ion. EDTA, a hexadentate ligand, is an example of a polydentate ligand that has six donor atoms with electron pairs that can be used to bond to a central metal atom or ion. Unlike polydentate ligands, ambidentate ligands can attach to the central atom in two places. A good example of this is thiocyanate, \(SCN^−\), which can attach at either the sulfur atom or the nitrogen atom.
Chelation is a process in which a polydentate ligand bonds to a metal ion, forming a ring. The complex produced by this process is called a chelate, and the polydentate ligand is referred to as a chelating agent. The term chelate was first applied in 1920 by Sir Gilbert T. Morgan and H.D.K. Drew, who stated: "The adjective chelate, derived from the great claw or chela (chely- Greek) of the lobster or other crustaceans, is suggested for the caliperlike groups which function as two associating units and fasten to the central atom so as to produce heterocyclic rings." As the name implies, chelating ligands have high affinity for metal ions relative to ligands with only one binding group (which are called monodentate = "single tooth") ligands. Both Ethylenediamine (Figure \(\PageIndex<2>\)) and Ethylenediaminetetraaceticacid acid (Figure \(\PageIndex\)) are examples of chelating agents, but many others are commonly found in the inorganic laboratory.
The chelate effect is the enhanced affinity of chelating ligands for a metal ion compared to the affinity of a collection of similar nonchelating (monodentate) ligands for the same metal.
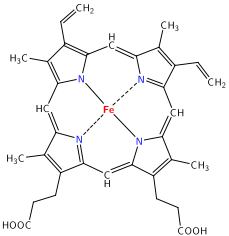
The macrocyclic effect follows the same principle as the chelate effect, but the effect is further enhanced by the cyclic conformation of the ligand. Macrocyclic ligands are not only multi-dentate, but because they are covalently constrained to their cyclic form, they allow less conformational freedom. The ligand is said to be "pre-organized" for binding, and there is little entropy penalty for wrapping it around the metal ion. For example heme b is a tetradentate cyclic ligand that strongly complexes transition metal ions, including Fe +2 in hemogloben (Figure \(\PageIndex\)). Some other common chelating and cyclic ligands are shown below:
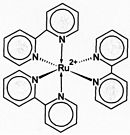
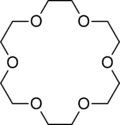
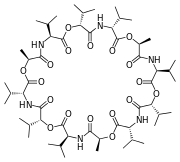
Figure \(\PageIndex<5>\): (let) 2,2'-Bipyridine, (center) 18-crown-6 2,2'-Bipyridine (right) valinomycin